Sumitomo Heavy Industries, Ltd. invests in Alpha Fusion Inc., developer of Astatine based radiopharmaceuticals for Targeted Alpha Therapy
March 07, 2023
Sumitomo Heavy Industries, Ltd. (“SHI”) (Head Office: Shinagawa-ku, Tokyo, President and CEO: Shinji Shimomura) announced the decision to invest in Alpha Fusion Inc. (“AF”) (Head Office: Kita-ku, Osaka, Chief Executive Officer: Tadashi Fujioka), a developer of Astatine based radiopharmaceuticals for Targeted Alpha Therapy (“TAT”).
AF will aim at establishing their pipeline (*1). Development of their radiopharmaceuticals is expected to be progressed by this funding and will lead more needs of Astatine-211. SHI is joining a new project led by Osaka University for mass production of Astatine-211 and will contribute for the development of the TAT, which is expected to be a future advanced medicine, with our technology of particle accelerators and a synthesis of radio-isotope(RI) labeled compounds.
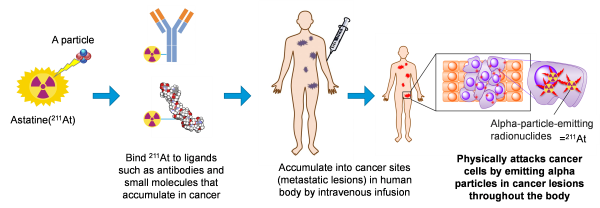
【TAT and Astatine-211】
TAT is a treatment that destroys cancer cells in the human body by injecting alpha emitting RI labeled candidate which selectively targets to cancer cells. Astatine-211(211At, Atomic number85, Half life time 7.2H) is an element belonging to halogens and has the property of emitting alpha particle. Due to no stable isotopes in nature, their properties have not been fully elucidated so far. However, it's getting attention in accordance with recent development of a nuclear medicine treatment and/or a theranostics (a new term combinated of therapy and diagnostic). Clinical studies had been already conducted in U.S. and Sweden etc. because of the advantages which is different from other approved RIs (177Lu, 223Ra, or 131I). The advantages are; stable production using naturally abundant bismuth (209Bi) with a relatively low-energy particle accelerator (cyclotron), short half-life time as 7.2 hours, and direct chemical formation with basic structure of candidates which conjugate with the disiese target site.
In Japan, investigator-initiated clinical trials targeting differentiated thyroid cancer(jRCT2051210144) at Osaka University and pheochromocytoma paraganglioma(jRCT2021220012) at Fukushima Medical University have started and further progress is highly expected.
【Image Diagram of TAT】
【Hiroyuki Tominaga, General Manager, Industrial Equipment Division, SHI】
There are highly expectations on TAT and the research and development is underway all over the world especially in Japan. Astatine-211 has the potential of easy delivery against to other alpha or beta emitted RI because of the production from a raw material abundant in nature by using a relatively low-energy particle accelerator (cyclotron). Therefore, I hope we’ll be able to make this new treatment widely available once the drug is approved. AF is a leading company aiming at a social implementation of TAT teaming up with Japanese companies and institutes. SHI would also want to do our best to contribute to TAT and its social implementation as one of the member of the team. And in the future, we would like to contribute to spread TAT from Japan to the world.
【Sunao Fujioka, Chief Executive Officer, AF】
AF is leading the world in the field of Astatine-211 radiopharmaceutical pipeline developments including clinical phase pipeline under the collaboration with Osaka university. SHI is an important business partner for establishing a supply chain for a stable delivery of the nuclide. And from this funding, AF will accelerate our business together with SHI in the stronger collaboration.
Through the social implementation and the commercialization of this innovative radiopharmaceuticals, AF aims to give hope to patients and their families around the world who fight with cancer and to provide a powerful tool to a healthcare professionals who also fight with cancer together with patients.
【Alpha Fusion Inc.】
AF is a startup company who is conducting a social implementation of Astatine-211 based on drug discovery transferred research results of Osaka university funded by Program on Open Innovation Platform with Enterprises, Research Institute and Achademia (OPERA) promoted by Japan Science and Technology Agency (JST). In the field of targeted alpha therapy, which has been rapidly attracting worldwide attention in recent years, Mission of the company is to let the Astatine drug development out by using its originality from Japan in the field of TAT that is rapidly attracting worldwide attention. AF aims to lead this innovative modality into the basic of cancer treatment and are aiming at the practical application of new cancer treatment by proceeding with pipeline research and development to business development on a world-class level.
| Alpha Fusion Inc. | Head office | 2-5-13, Umeda, Kita-ku, Osaka, Japan |
| Board of director | CEO, Sunao Fujioka | |
| Business domain | Technical research, drug discovery development, and business development of astatine-introduced compounds into a wide range of small molecules and antibodies, including Sodium astatide | |
| Capital | 251 MJPY | |
| Web site | https://alpha-fusion.com/ |
(*1) Pipelines of AF are followings. Please refer to the URL for detail.
●Pipeline 1 : Drug for thyroid cancer
( Under Phase I clinical trial : https://prtimes.jp/main/html/rd/p/000000001.000091191.html)
●Pipeline 2 : Drug for prostate cancer
( Preparing for clinical trial in next year : https://prtimes.jp/main/html/rd/p/000000003.000091191.html)
●Other 4 drugs are under development and preparing for a patent application for the basic technology
