Notice Regarding Our First Overseas Introduction of BNCT in the Hainan Medical Tourism Pilot Zone, China
June 24, 2022
Sumitomo Heavy Industries, Ltd. (Head office: Shinagawa-ku, Tokyo; President: Shinji Shimomura; hereinafter “Sumitomo Heavy Industries”) and STELLA PHARMA CORPORATION (Head office: Chuo-ku, Osaka; President: Koki Uehara; hereinafter “Stella Pharma”) have respectively entered into a sales contract for BNCT System, “NeuCure®“as well as “NeuCure® Dose Engine”, the BNCT dose calculation program and master service agreement for a boron drug for BNCT, “Steboronine®”, with China Biotech Services Holdings Limited, a company listed on The Stock Exchange of Hong Kong Limited, (Stock Code: 8037) and its subsidiary, Pengbo (Hainan) Medical Technology Co., Ltd. (hereinafter “Pengbo Co., Ltd.”), regarding the introduction of BNCT in Hainan Boao Lecheng International Medical Tourism Pilot Zone in China (hereinafter “Hainan Medical Tourism Pilot Zone”).
The Hainan Medical Tourism Pilot Zone is a special deregulated zone aimed at promoting medical tourism, with priority given to import of medical equipment and drugs.
A distinctive feature of the Hainan Medical Tourism Pilot Zone is that, similar to Japan, the use of any medical equipment or drugs in China require prior testing via clinical trials before applications can be filed. However, unlike the normal process of obtaining approval from the regulatory authorities, this system will grant import licenses for medical equipment and drugs that have already been approved in other developed countries on the premise that they are clinically urgently needed at designated medical institutions, even if they have not yet been approved in China. This therefore allows the medical institutions not to conduct clinical trials and to use such medical equipment and drugs for clinical treatment.
This time, the only globally approved BNCT, combination of NeuCure® and Steboronine® will be introduced in China through this system.
Pengbo Co., Ltd. will obtain a Medical Institution Practicing License and establish a designated medical institution (hereinafter “BNCT center”) in the Hainan Medical Tourism Pilot Zone. The land for the planned construction site has already been acquired, and the provision of BNCT is scheduled to start from FY2025 after the construction of the BNCT center and the installation of the BNCT system, “NeuCure®”, are completed.
The disease indicated for BNCT is “locally unresectable recurrent or unresectable advanced head and neck cancer” (hereinafter, “local head and neck cancer”), which is already approved in Japan. The total number of head and neck cancer patients in China is estimated to be around 140,000 per year. We will continue to develop BNCT as a new cancer treatment option for overseas patients who are waiting for it to be indicated as well.
If a new indication for BNCT is approved in Japan in the future, the treatment will also be made available in the Hainan Medical Tourism Pilot Zone similar to local head and neck cancer. In addition, the real world data obtained at the BNCT center in the Hainan Medical Tourism Pilot Zone can be used to apply for approval in China if the management, research, analysis, and evaluation are conducted in accordance with relevant regulations. This will help us to promote BNCT in the Hainan Medical Tourism Pilot Zone and use it as a foothold to expand BNCT into China.


| Overview | Comprises Hainan Island situated at the southernmost tip of China and surrounding islands. It is the province with the smallest land area and the largest jurisdictional sea area in China. (Area: 354,000 km²; population: 9.7438 million people) |
|---|---|
| Main industries | Includes tourism, marine shipping, and agriculture. |
| GDP | RMB 647.52 billion |
| Total import & export value | RMB 147.678 billion |
【BNCT (Boron Neutron Capture Therapy)】
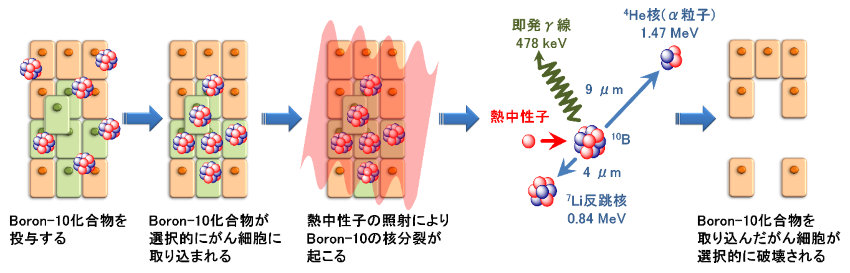
Boron-neutron capture therapy (BNCT) is a type of radiotherapy where the boron-containing drug for BNCT (Boron-10) is administered to cancer patients, which causes the selective uptake of boron (Boron-10) in cancer cells. A low-energy neutron beam is then irradiated on the cancerous area from outside the body. When the neutrons collide with the boron (Boron-10) in the cancer cells, nuclear fission occurs. This in turn generates alpha particles (helium nuclei) and lithium recoil nuclei (lithium nuclei) that damages cancer cells. These charged particles have a respective range of only about 9 µm and 4 µm in the body, which is approximately the size of a single cell. In theory, these characteristics allow us to selectively destroy cancer cells that have taken up boron (Boron-10) at the cellular level, with little to no damage to the surrounding normal cells.
【BNCT system: NeuCure®】
The NeuCure® system is a medical neutron irradiation machine that uses high current 30MeV proton AVF-cyclotron and a beam control system based on Sumitomo Heavy Industries’ development experience in cyclotrons for physics research and medical applications as well as beam control technologies accumulated over nearly 50 years. Sumitomo Heavy Industries has been developing the NeuCure® system together with the Institute for Integrated Radiation and Nuclear Science, Kyoto University (Kumatori-cho, Sennan-gun, Osaka), since 2007. Beryllium is used for the target that converts the proton beam produced by the cyclotron into neutrons. It features excellent safety and stability required by medical institutions, making it possible to install the system in a hospital.

【A boron drug for BNCT: Steboronine®】
Steboronine®, a product developed by Stella Pharma, is manufactured using “Borofalan (10B)” as the active pharmaceutical ingredient, which contains highly purified (>99%) Boron-10 that is required for BNCT. As the structure of Borofalan (10B) is similar to that of phenylalanine or tyrosine, which are essential amino acids, it is taken up by cancer cells with high amino acid requirements via LAT-1, an amino acid transporter unique to cancer cells. This allows Boron-10 to selectively accumulate in cancer cells, which will lead to greater effect when the cells are irradiated with neutrons.
| Sumitomo Heavy Industries, Ltd. | Location | 1-1 Osaki 2-chome, Shinagawa-ku, Tokyo |
| Title & Name of Representative | Shinji Shimomura (President) | |
| Business Description | Manufacturing and marketing of various types of industrial machinery, from general industrial machinery to precision control machinery | |
| Capital | JPY 30. 872 billion | |
| Corporate website | https://www.shi.co.jp/english/index.html | |
| STELLA PHARMA CORPORATION | Location | 3-2-7 Kouraibashi Chuo-ku, Osaka |
| Title & Name of Representative | Koki Uehara (President) | |
| Business Description | Development, manufacturing and marketing of boron drug used for BNCT | |
| Capital | JPY 3.8 billion | |
| Corporate website | https://stella-pharma.co.jp/en/ | |
| China Biotech Services Holdings Limited | Location | Suites 1904-5A, 19/F, Sino Plaza, 255-257 Gloucester Road, Causeway Bay, Hong Kong |
| Title & Name of Representative | Mr. Liu Xiaolin (Chairman) | |
| Business Description | Precision Diagnosis、Health Products | |
| Capital | HKD 587.015 million | |
| Corporate website | http://www.cbshhk.com/ | |
| Pengbo (Hainan) Medical Technology Co., Ltd. | Location | No. 32 Kangxiang Road, Hainan Boao Lecheng International Medical Tourism Pilot Zone, Qionghai City, Hainan |
| Title & Name of Representative | Ms. Zhao Dan | |
| Business Description | Medical services, medical cosmetic services | |
| Capital | RMB 150 million |
