Sumitomo Heavy Industries, Ltd. obtains medical device approval for manufacturing and sales of accelerator based BNCT system and the dose calculation program in Japan. - World’s first BNCT systems as medical device -
March 12, 2020
TOKYO-Sumitomo Heavy Industries, Ltd. (TOKYO:6302)(President: Shinji Shimomura, referred as SHI hereinafter) has performed clinical trial of the BNCT*1) (Boron Neutron Capture Therapy) system using an accelerator (cyclotron), targeting carcinoma of the head and neck region in collaboration with STELLA PHARMA CORPORATION (President: Tomoyuki Asano). And SHI has applied the system for new medical device approval on 11th October, 2019. Today, we would like to announce that Sumitomo Heavy Industries, Ltd. obtains new medical device approval for manufacturing and sales of accelerator based BNCT system (NeuCure™ System) and the dose calculation program (NeuCure™ Dose Engine) from Japanese Ministry of Health, Labor and Welfare. These are the world’s first medical devices for BNCT.
Approved products
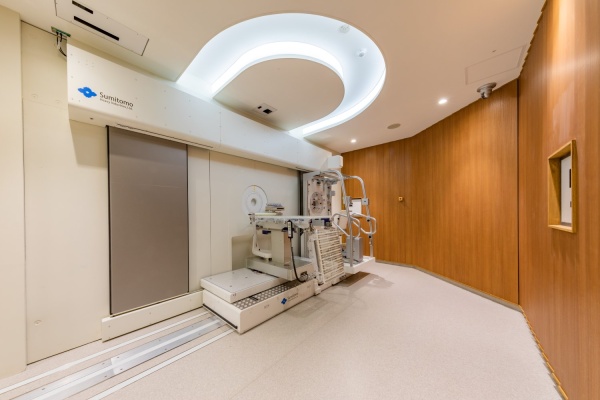
BNCT System NeuCure™
This system is a neutron irradiation system used for BNCT.
BNCT dose calculation program NeuCure™ Dose Engine
This program is a program used for dose distribution calculation of BNCT to support the treatment planning.
These products have been approved for unresectable locally advanced or locally recurrent head and neck carcinoma. SHI will also apply these products for recurrent malignant glioma that is also designated as SAKIGAKE designation system *5).
These products shall be used in combination with the medicine below.
Generic Name: Borofalan [10B], Product Name: Steboronine® (10B) Intravenous drip bag 9000 mg/ 300 mL
* This medicine has been recommended of approval by Ministry of Health, Labor and Welfare as the result of deliberation in Second Committee on Drugs on 26th, Feb., 2020.
Features of products
BNCT System NeuCure™
This system uses high current 30MeV proton AVF-cyclotron based on 50-year technical development experience of SHI for medical and physics research fields. SHI has been developing NeuCureTM system since 2007 together with Institute for Integrated Radiation and Nuclear Science, Kyoto University. *2) Beryllium is used for the translation from proton to neutron in order to realize safety and reliable system which shall be required by hospitals. And NeuCure™ system has become the world’s first medical system which can be installed in hospitals.


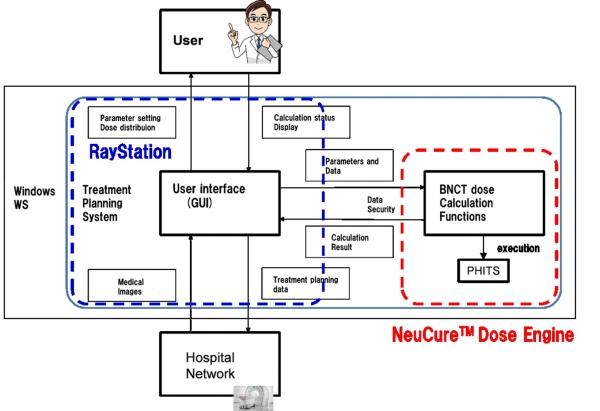
BNCT dose calculation program NeuCureTM Dose Engine
Dose calculation of BNCT essentially requires Monte Carlo method which enables to reproduce reactions between neutron and atoms in human body in detail. NeuCureTM Dose Engine uses PHITS code which was developed by Japan Atomic Energy Agency *3). SHI has developed additional functions enabling BNCT dose calculation and data I/F including data security. General functions as a treatment planning system such as; image importing, plan creation, contouring, plan evaluation, and reporting, etc. are provided by means of the combination with RayStation *4), which is a product of RaySearch laboratories, AB.
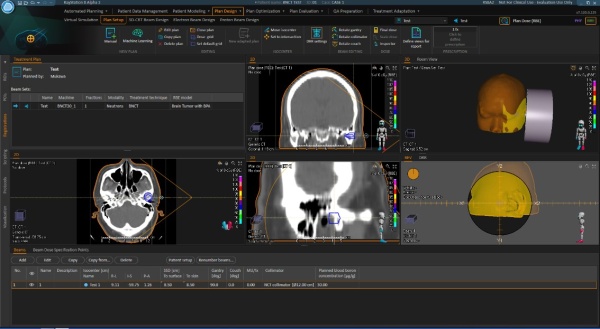
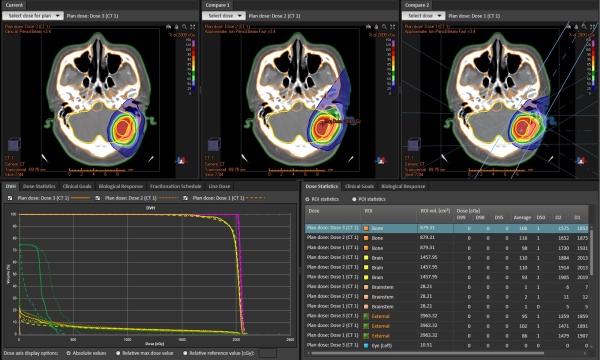
Market and SHI’s target
BNCT is unique radiation therapy method which is led by Japanese researchers. SHI believes better treatment will be provided to patients in the world. Once STELLA PHARMA CORPORATION obtains the drug application approval, SHI would like to develop BNCT market globally together with partners by means of sending information of BNCT and the clinical outcome to the world. It may take years for obtaining global common recognition, however, SHI would like to install more than 100 systems globally as a long term target. SHI will keep making efforts to improve and develop NeuCure™ system and NeuCure™ Dose Engine.
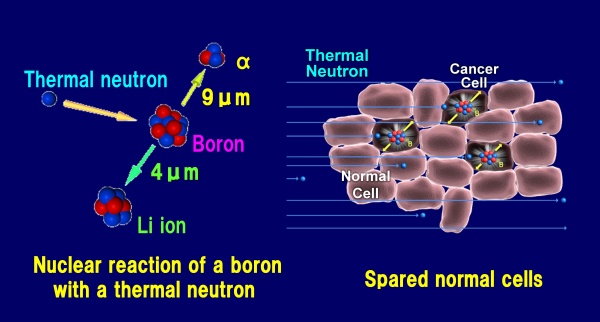
*1 BNCT is a type of cancer radiotherapy. In this therapy method, a boron medical agent for BNCT is given to cancer patients whereby cancer cells selectively absorb boron (Boron-10). The results is that low-energy neutrons are radiated which in turn causes fission of the boron (Boron-10) nuclei inside the body. This reaction releases α particles (helium nuclei) and Li recoil nuclei (lithium nuclei), which possess enough energy to damage cancer cells. The range of these charged particles is approximately 9μm and 4μm respectively. This range is approximately the size of a cancer cell. This makes it possible to selectively destroy cancer cells, which have absorbed the boron (Boron-10), while minimizing damage to surrounding healthy cells.
*2 SHI has been developing accelerator based BNCT system together with Integrated Radiation and Nuclear Science, Kyoto University since 2007. In 2009, SHI installed the first system in the institute. In 2015, SHI installed the second system in Southern Tohoku Hospital, Fukushima, and in 2019, SHI installed the third system in Osaka medical college, Osaka. On the other hands, SHI started phase II clinical trial in 2016 together with the partners following preclinical and phase I clinical trial. In 2017, this project was specified as one of the subjects for SAKIGAKE Designation System.
*3 PHITS (Particle and Heavy Ion Transport code System) is well known Monte Carlo calculation code developed by Japan Atomic Energy Agency (JAEA). It has many references of research for the simulation of various radiation behavior and it has more than 4,000 users globally,
*4 RayStation is an advanced treatment planning system which has been marketed by RaySearch laboratories, AB (Stockholm, Sweden), who is a medical technology company that develops advanced software solutions for improved radiation therapy of cancer. RayStation is used at clinics all over the world in good reputation because of its flexible platform with advanced tools including optimization tool. RaySearch’s products are distributed through licensing agreements with leading medical technology companies. RaySearch’s software is used by over 2,600 clinics in more than 65 countries. RaySearch was founded in 2000 as a spin-off from the Karolinska Institute in Stockholm
*5 SAKIGAKE Designation System was implemented in 2015 as a strategic package supported by the Japanese government to speedily promote practical applications of innovative pharmaceuticals, medical-devices and regenerative medicine products for life-threatening diseases in Japan. The goal is commercial implementation of this treatment method before it is available globally by covering the entire process, including research, clinical trials, safety responses, insurance applications and international deployment. This project was specified as one of the subjects for SAKIGAKE Designation System on February 29, 2017, under the name of “Boron Neutron Capture Therapy (BNCT) system”.
