
Cancer Therapy System "Boron Neutron Capture Therapy (BNCT system)"
Sumitomo Heavy Industries developed a compact accelerator used for a boron neutron capture therapy (BNCT) system by utilizing the know-how had been accumulated through developing medical cyclotrons and their beam control systems. After over a decade of efforts, the BNCT radiotherapy system NeuCure® and the BNCT dose calculation program NeuCure® Dose Engine finally received regulatory approval as a new medical system. However, various problems and challenges had been lain ahead of this unprecedented project before the regulatory approval.

Medical Strategy Group,
Medical and Advanced
Equipment Management Dept.,
Industrial Equipment Div.

Engineering Physics Dept,
Technology Research Center
Corporate Technology
Management Group

Medical Strategy Group,
Medical and Advanced
Equipment Management Dept.,
Industrial Equipment Div.
Essential item for project implementation, Selecting appropriate collaborative development partners on a reasonable contract basis

After receiving regulatory approval as a new medical system in March 2020, referred to below as BNCT system (the following, BNCT) would be covered by health insurance in June of the same year. This breakthrough cancer treatment method that combines medical agents and radiation therapy destroys cancer cells selectively. As a result, BNCT has brought breakthrough innovations to the cancer treatment field, which downsized equipment and reduced patient burden.
"BNCT originally performed with a treatment planning system for research purposes. However, a revision of the medical law redefined the treatment planning system as the part of a medical device. That's why we ended up developing the software as a medical device for the first time even as an equipment manufacturer, " says Naoaki Tanizaki of the Industrial Equipment Div. Sumitomo Heavy Industries implemented this project with collaborative development partners, including the Japan Atomic Energy Agency, Kyoto University, and STELLAPHARMA. As the facilitator representing Industrial Equipment Div., Tanizaki is responsible for domestic and overseas coordination.
"As a machinery manufacturer, we have no sense of creating program interfaces as you would see at hospitals, unfortunately (with a wry smile). Because Operability and Functionality is very important for medical programs, we selected an external company to develop the critical portions for us on a contract basis."
However, due to our limited development costs and time, we could not accept all the conditions the company would propose. The team also seemed to have some struggles behind the scenes when obtaining internal approval.
Tanizaki says, "In the final stage of the contract, I was told by directors to review the specifications further to make them commensurate with the contract amount. I thought management's argument was reasonable because this project costs as much as several hundred million yen for a single clinical trial. So, we negotiated with the partner concerning the division of roles in development and post-sales support items, which led to a successful contract."
Clinical trials of BNCT started in 2007. Selling BNCT as a medical system required us to submit necessary data on design, specifications, and clinical trials to relevant authorities (the Ministry of Health, Labour and Welfare and its subordinate organization, the Pharmaceuticals and Medical Devices Agency, hereinafter, PMDA) for obtaining their regulatory approval.
"We can sell ordinary mechanical devices after they pass our internal tests. However, a medical system typically takes nearly a year from its application submission to regulatory approval. It takes six months even where a special provision* is applied. During this period, we needed to explain everything to the authorities day after day."
*The Ministry of Health, Labour and Welfare's SAKIGAKE Designation System (conducting priority consultations, prior assessment, and priority reviews)
We were allowed to sell BNCT after obtaining regulatory approval for the medical system. Still, many issues remain unresolved, including improvements in BNCT calculation speed, equipment downsizing, and price reductions.
"We developed BNCT by prioritizing safety, stability, and reliability as a medical system. This stance will not change even if we face competition in the domestic and overseas markets. While our competitors are developing similar products and conducting their clinical trials, we will accumulate the operational know-how of the BNCT with our customers. We are also passionate about offering a better system by adding new features to this BNCT sooner than anyone else."
The use of the BNCT is limited to unresectable cancer, meaning locally advanced or locally recurrent head and neck cancers, with many expecting the treatment to be used for other types of cancers in the future.
"Of course, adding a new treatment application site would require applying to the authorities for regulatory approval each time, requiring close cooperation with our partners. Doing it again would require an enormous amount of time and money. Without the promise of recovery in investment, no one - us, hospitals, or partners - wants to move forward. Under this circumstance, we will build up our clinical track records so the BNCT cancer treatment will become more popular."
Development to reduce the burden on the medical field and patients

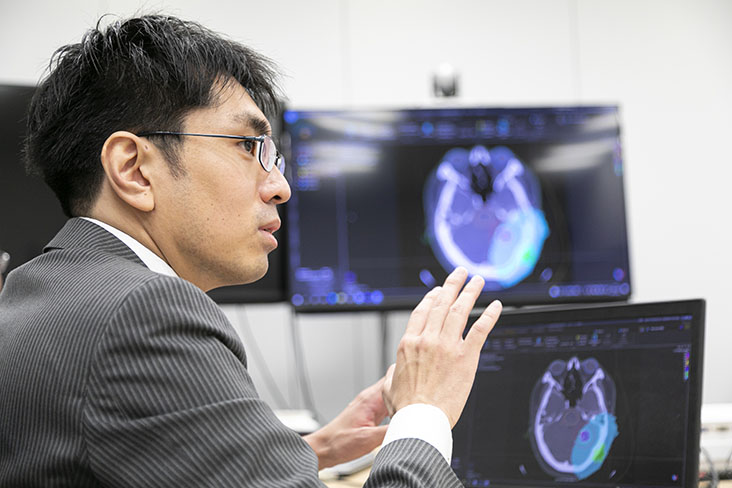
Performing BNCT requires precise dose calculation in advance for the targeted tumor area and its surrounding normal tissue. In our case, BNCT dose calculation is conducted using domestically developed "Monte Carlo Codes." As a development team member, Tetsuya Mukawa of the Technology Research Center, Corporate Technology Management Group, scrambled around carrying a large laptop computer.
He says, " I had studied BNCT with Monte Carlo codes at university. And I joined the company in 2014, just as the development of the treatment planning system was starting. At that time, BNCT clinical trials had already started."
He thoroughly had researched existing treatment planning systems used in general radiation therapy and selected an overseas software maker as our potential partner for this project. Only this survey took him six months. Furthermore, it took him one more year to study the specifications that can add BNCT features to candidate software enabled for BNCT.
He says, "My main tasks included building a system to reproduce our irradiation system using Monte Carlo Codes, developing specifications for how to implement the irradiation on the interface, and communications with overseas software makers. There were many cases where verbal explanations did not work well for overseas staff. So, I sometimes tried to make them understand by forcibly implementing a prototype in the system (smiling)."
Clinical trials were conducted using software for research purposes. The application of the program to obtain regulatory approval for would be approved by showing its equivalence with the program for clinical trials. However, the program was used first at a hospital after the completion of clinical trials and regulatory approval.
"I went to the hospital to watch the first cancer treatment feeling frightened. Two years has passed after the release of the medical system, we have begun to identify various challenges that need to be addressed. In addition to the increase in the dose calculation speed, there may be a need to increase the variation of user-friendly displays. In medical technology, new evaluation techniques constantly emerge one after another. Therefore, how we must keep up with them."
BNCT therapy planning takes a lot of time because the dose distribution needs to be calculated in minute detail by virtually flying tens to hundreds of millions of neutrons and electromagnetic waves and then reacting them with boron and other elements in the body. Precise computation inevitably requires extended time.
"The computer's processing speed is insufficient, and the software has room for more optimization. There are other requests from the customers, and we will continue to develop better software by screening what is really needed. If we try to satisfy all requests, the software will become incomprehensible, which is why we need to draw a line."
Advancements in computers and software have finally enabled the use of BNCT at clinical sites. But, accelerator-based BNCT has just begun for clinical cancer treatment. We believe that the equipment and program will need further improvement and refinement in the near future. Our immediate goal is to demonstrate an overwhelming difference in performance to keep our competitors at bay.
"I am aiming to shorten the calculation time. In addition to developing software, I also want to develop related equipments, including applications.
BNCT has a low recognition today, so I set the goal of enhancing the recognition of BNCT to the level everyone knows. I believe the role of a machinery manufacturer like us is to keep discussion with doctors and develop a new device when the need arises."
Days spent for explanation to the authorities for application approval

"I have been involved in BNCT since the beginning of its development. I was responsible for the system design at that time. After joining a team working on clinical trials, I became a member of a team dedicated to regulatory affairs. My experience in design was very helpful when facilitating the application process and when I needed to explain to the authorities."
These are the comments of Yuji Kikuchi of the Industrial Equipment Div. BNCT, a machine that combined radiation and agents, a breakthrough system that no one had ever experienced before. For that reason, it was essential to have close collaboration with a medical agent manufacturer.
Kikuchi says, "Decades ago, the company manufactured a product that utilized a combination of drugs and a machine for intended purposes. However, the combination of radiation and medical agents was brand new to us, and there was nobody who knew the past. And this project was entirely new for both the company and employees. We are machine experts but have just started learning about medical agents. We learned a lot of know-how from medical agent manufacturers, such as how to deal with pharmaceutical regulations and the authorities, based on which we built up the knowledge needed for this development. We needed to discuss how to write application documents many times, partly with the help of consultants."
At that time, the BNCT device e and medical agents were applied to a subject of the SAKIGAKE Designation System, which started in 2015. Once designated, the project was treated preferentially in the examination and consultation processes, shortening the time needed for examination to about six months from one year. For the authorities, BNCT, the world's first cancer therapy system, was a centerpiece of the SAKIGAKE Designation System.
"Since about two months before the regulatory approval, the authorities had begun to call Mukawa-san and myself frequently every day. The PMDA is a subordinate organization of the Ministry of Health, Labour and Welfare. We had explained the application to the PMDA, and the PMDA would explain to the Ministry of Health, Labor and Welfare instead. We emphasized the safety and efficacy of BNCT to the authorities, and they listened and sincerely responded to us. We repeated this process many times to ensure their complete understanding."
There were other various standards to satisfy to obtain regulatory approval, and the examination process proceeded at the pace of the authorities. Kikuchi had spent very hard time to keep up with the fast pace.
"The law stipulates various standards we must satisfy to get our product approved as a medical system. There are many items to check, including mechanical, electrical, radiation, and biological safety. After applying, you are expected to thoroughly explain everything to the authorities until you can confidently state everything is alright. The final application documents to explain everything to the authorities filled with two cardboard boxes. The call informing us of the approval triggered a memory I would want to forget to run through my head: I was walking back alone to my room in a short-term apartment in heavy snow late at night after completing the data measurement needed for the application (smiling). Through this project, I was involved in the entire process of the development and approval of a medical system. From the bottom of my heart, I think it was a valuable experience, including my interactions with the authorities."
The application work was performed at yearend. So, Kikuchi could take a short New Year's break. But ....
"After the break, an Expert Committee would hold where experts discuss the pros and cons of approval. While I was off duty, I had been worrying about how things would be going after the break, and it made me uneasy. As it turned out that , the PMDA explained the details to the Ministry of Health, Labour, and Welfare without any problems, it meant the green light was ON to move on to the next stage. That moment was reward for the enormous amount of base data and the countless interactions with responsible persons. In looking back these harder time, I seldom felt stress and could be absorbed in the project in a good sense."
The BNCT device is expected to become the more appealing product with the addition of new features. The BNCT device with some modifications received regulatory approval this February, and the user hospital truly appreciates the performance.
"The increased flexibility of patient body position settings helped reduce the burden" on patients during treatment. We will continue to proactively improve the device. My team is expected to apply the ideas as soon as possible, which the R&D team comes up with based on user feedback as soon as possible and obtain the regulatory approval smoothly. Compared with an ordinary machine, a modified BNCT device requires more time and effort to be used at medical site. My intent to make it a more attractive product remains unchanged since the days when I was responsible for the design."
Getting BNCT approved is not the goal but the second starting line. The challenge to make BNCP cancer therapy more accessible and save more patients has just begun.
■ Disclaimer
The information on the medical system described in this document is intended to disclose the background of its development. It is in no way promotion or advertisement.
*All contents are as of the time of the interview.

SHI Pride
- Vol.1 A Clear View of the Distant Heavens
- Vol.2 Cures without Surgery: Advanced Cancer Treatment
- Vol.3 Exploring the Mysteries of Space
- Vol.4 Dramatically Changing Quayside Cargo Handling
- Vol.5 Cyclo® Drives Continue to Evolve
- Vol.6 The Steam Turbine - Protecting the Environment and Advancing Society
- Vol.7 Persistent Determination to Reduce Defects, Losses and Faults to Zero
- Vol.8 Treating Cancer through the Use of Neutrons - A Compact Accelerator Opens the Door to Widespread Use -
- Vol.9 Automated storage system with unrestricted movement, for transforming the future of the logistics industry
- Vol.10 Anaerobic Wastewater Treatment and Biogas Power Generation System using Ume Seasoning Effluent bringing Recycling-Friendly Society
- #01 Technology that has the potential to dramatically change automobile frame manufacturing STAF created by maintaining a comprehensive customer perspective
- #02 The ECY Series:A smaller Gear reducer after over 10 years of development
- #03 Cancer Therapy System "Boron Neutron Capture Therapy (BNCT system)"
